Menu
- News
- IFAA
- Committees and Programmes
- Societies
- Plexus
- IFAA Congresses
- Recommendations
- Archive of relevant IFAA documents
close
In 2012 the IFAA published guidelines to support good practice around the use of human bodies and tissues for anatomical purposes. Continued development of technologies since the original guidelines, with the now ubiquitous use of the internet and digital technologies, means there are now additional considerations which require ‘best practice’ guidance for the anatomy community. One of these considerations is image acquisition and use, a topic that was originally referred to in a limited capacity as ‘Item 6’ in the 2012 guidelines. This topic is here expanded to provide guidelines that are more congruent with the contemporary education and research environment. The term ‘images’ refers specifically to photographs, videos, and images of actual human tissues as well as those generated by modalities such as ultrasound, computed tomography, and magnetic resonance imaging.
Images that arise from human tissues are not physical specimens, but their representations. Nevertheless, they are derived from actual persons and therefore deserve special consideration in regard to their acquisition, storage and use. The use and distribution of images in ways that are not considered ethical can undermine the relationship with local communities, and this necessitates reflection around how these resources are managed.
It is acknowledged that access to donated bodies is not globally universal, and where these are not available yet there may still be a reliance on unclaimed bodies. If unclaimed, unconsented bodies are used, the spirit of the document should still apply and institutions should aspire to meet the guidelines, even though this may mean that fulfilling the requirements of some sections of the guidelines is not possible (such as sections 2, 3, and 4).
1. The use of images derived from human tissues should be considered as part of the respectful treatment of those whose bodies are used in anatomy education and research. This treatment acknowledges that their use should be restricted to defined purposes in education or research and that anatomical dissection of deceased individuals should remain confined to a protected non-public space.
2. The use of images should be covered by informed consent of the donating individual that is appropriate for the intended outcome, use or purpose of the image (see figure). For example, where applications or use of images are more likely to be applied in instances such as commercial use, then specific consent for that purpose is required. In instances such as images of histological specimens, less specific consent is suggested as being adequate.
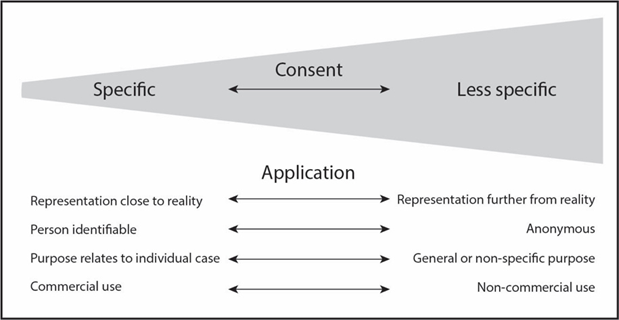
3. Informed consent for anatomical body donation should include information pertaining to images of a donor’s body (or part thereof) being used for education and / or research purposes. Contained within the information provided to potential donors should be details around image acquisition, storage, duration and manner of use. If images may potentially be distributed to other institutions for educational and / or research purposes, this should be described on the information and consent forms.
4. Informed consent documentation should include a statement that any images that could lead to the identification of the person must be specifically consented by the donors and / or their loved ones or legal representative. In general, identifying marks, accession numbers, or other features that may potentially lead to identification should be redacted from images.
5. Images of human tissues acquired from deceased individuals should never be used on social media or other non-password protected internet sites. Use on restricted-access anatomy-related websites such as institutional platforms is acceptable. These limitations are necessary to uphold the dignity of the individuals, avoid bringing the dissection of donors into a non-protected public space, and to prevent donated bodies being misused or abused for non-academic purposes (including morbid curiosity).
6. The development of educational resources that contain images of human tissues should seek to embed these images within documents so that files of individual images are not able to be acquired by those using the documents. Where online education is delivered, students and others must not copy or ‘screen-shot’ images of human tissues. This is to prevent ad hoc distribution of individual images that are not associated with an educational context.
7. Images of human tissues should not be commodified or commercialized, meaning they should not be bought, sold, or traded for profit. As per Item 2 of the 2012 guidelines, some exchange that allows support for real costs incurred is considered appropriate. If images are to be used in the development of commercial anatomical resources, such as textbooks or other educational products, the donor must specifically consent to image use for this purpose, and, where possible, the donor’s loved ones should also be made aware of this use at the time of consent. Specific consent for this purpose should be made with documents that are independent of the standard consent processes, so any educational purpose that may have commercial sequalae is not viewed as being part of the standard consent practice.
8. Those who develop applications or resources from images of human tissues, including commercial educational systems such as image projection tables or virtual anatomy systems, must disclose the sources of their material (i.e. data sets) and their consent status.
9. Where acquiring or using images of human tissues is possible, or where technologies are used that include images of human tissues, faculty, staff and students at these institutions should receive instruction on contemporary ethical practices around image acquisition, storage and use.
10. Areas where human tissues are used should have clear and appropriate signage that indicates no unauthorized photography or image capture is to take place. This is to prevent the ad hoc acquisition of images by faculty, students, staff, or other parties.
11. Images of human tissues should only be acquired from sources where the status of donor consent is able to be verified. This includes images acquired randomly from the internet, and from commercial educational systems that do not disclose the sources of their images. A ‘best practice’ is for images to be generated locally from fully consented donors or to be acquired from other institutions where donors were fully informed and consented for such use. Use of historical image data sets acquired prior to the development of contemporary ethical standards should be undertaken with care, and with reference to these guidelines to facilitate appropriate decision-making about their use. In these cases transparency is a priority and it may be useful to provide indication on whether the consent status of the person was unknown, unconsented, or consented. Where possible, images from historical collections from unknown or unconsented persons should be replaced with images from consented persons, except for educational settings that specifically address the history and ethics of their acquisition.
12. All digital images of human tissues acquired by an institution for education and / or research purposes must be stored on secure, password protected devices that are only accessible to designated faculty, staff and students at that institution. To maintain security and control of image databases, and to facilitate access for the purposes of an audit, it is a ‘best practice’ that all images of human tissues be stored locally and not on non-institutional, commercial servers.
13. If images of human tissues are not being specifically and consistently used for an educational and / or research purpose anymore, it should be considered to destroy them after a reasonable period to avoid the accumulation of images of human specimens detached from a specific purpose.
14. Anatomical Oversight Committees (AOC) for the institution or donor program, or the committee or persons directly responsible should an AOC not be established, should assist in the development of, and provide approval for, the processes and / or policies around human image acquisition, storage use and destruction. AOC should also consider how donors could be acknowledged in resources or final products. It is a ‘best practice’ for these processes and policies to be transparent and discoverable.
This set of guidelines supplements the IFAA ‘Recommendations of Good Practice for the Donation and Study of Human Bodies and Tissues for Anatomical Examination’ (2012).
Developed by:
The Federative International Committee for Ethics in the Medical Humanities (FICEM) for the International Federation of Associations of Anatomists (IFAA)
April/July 2023
Web design and maintenance by michaelnissen IT